The equilibrium constant of a chemical reaction. Chemical equilibrium: chemical equilibrium constant and ways to express it The chemical equilibrium constant does not depend on
Chemical equilibrium is a state of a chemical system in which one or more chemical reactions reversibly proceed, and the rates in each pair of forward-reverse reactions are equal to each other. For a system in chemical equilibrium, the concentrations of reagents, temperature and other parameters of the system do not change with time
A2 + B2 ⇄ 2AB
A quantitative characteristic of chemical equilibrium is a quantity called the constant of chemical equilibrium.
At a constant temperature, the equilibrium constant of a reversible reaction is a constant value showing the ratio between the concentrations of the reaction products and starting substances, which is established at equilibrium.
The equilibrium constant equation shows that under equilibrium conditions, the concentrations of all substances involved in the reaction are interconnected. A change in the concentration of any of these substances entails a change in the concentration of all other substances. As a result, a new concentration is established, but the ratio between them corresponds to the equilibrium constant.
58. Factors that determine the direction of chemical reactions. Chemical processes must proceed in the direction of decreasing the internal energy of the system, i.e. in the direction corresponding to the positive thermal effect of the reaction.
The second factor influencing the direction of chemical reactions is the principle of the direction of processes to the most probable state, i.e. in chemical reactions, due to the principle of directing processes to a minimum of internal energy, atoms combine into molecules, during the formation of which the greatest amount of energy is released.
The tendency to transition to the state with the lowest internal energy is manifested at the temperature to the same extent. The tendency to achieve the most probable state becomes stronger the higher the temperature. At low temperatures, in most cases, only the influence of the first of these tendencies has a practical effect, as a result of which exothermic processes proceed spontaneously. As the temperature rises, the equilibrium in chemical systems shifts more and more towards the reaction of decomposition or an increase in the number of states of atoms. In this case, each temperature corresponds to an equilibrium state, characterized by a certain ratio of the concentration of reactants and reaction products
59. Displacement of chemical equilibrium. La Chatelier's principle. If the system is in a state of equilibrium, then it will remain in it as long as the external conditions remain constant. Of greatest importance are cases of violation of chemical equilibrium due to a decrease in the concentration of any of the substances participating in the equilibrium; change in pressure and temperature. These imbalances are governed by the Le Chatelier principle: if a system in equilibrium is affected, then as a result of the processes occurring in it, the equilibrium will shift in such a direction that the impact will decrease.
60. Gibbs phase rule. For any system in equilibrium, the sum of the number of phases (P) and the number of possible states of the system (V) is greater than the number of components (C) by 2: P + V = C + 2
61. Solutions. dissolution process. A solution is a solid, gaseous or liquid homogeneous system consisting of two or more components, the amount of which can vary over a wide range. The most important type of solutions is liquid. Any solution consists of solutes and a solvent, i.e. environment in which these substances are evenly distributed in the form of molecules or ions. Usually, a solvent is considered to be that component that exists in its pure form in the same state of aggregation as the resulting solution.
The homogeneity of solutions makes them very similar to chemical compounds.
The difference between a solution and chemical compounds is that the composition of a solution can vary over a very wide range. In addition, many properties of its individual components can be found in the properties of the solution, which is not observed in the case of chemical compounds.
The variability of the composition of solutions brings them closer to mechanical mixtures, but they differ sharply from them in their uniformity. Solutions occupy an intermediate position between mechanical mixtures and chemical compounds.
The separation of molecules from the surface of the crystal during dissolution is caused, on the one hand, by their own thermal vibrations of the molecules, and, on the other hand, by the attraction of molecules by the solvent.
A solution in equilibrium with a solute is called a saturated solution.
62. Ways of expressing the composition of the solution. a) Mass fraction: ω \u003d m 1 / (m 1 + m 2) * 100% where m 1 is the dissolved substance; m 1 + m 2 - the mass of the solution; m 2 is the mass of the solvent;
b) Mole fraction N= ν 1 / ν 1 + ν 2 - this is the ratio of the number of moles of a solute to the sum of the amount of all substances that make up the solution;
c) Molar concentration C \u003d V 1 / m 2 - the ratio of the amount of substance contained in the solution to the volume of the solution (mol / l);
d) Mole concentration C \u003d V e1 / V - the ratio of the amount of substance contained in the solution to the mass of the solvent (mol \ kg);
e) The molar concentration of the equivalent is the ratio of the amount of the equivalent substance contained in the solution to the volume of this solution (mol / l).
63. Solubility, Henry's Law. Solubility is the ability of a substance to dissolve in a particular solvent. A measure of the solubility of a substance under given conditions is its content in a saturated solution. However, as a rule, substances consisting of polar molecules and substances with an ionic type of bond dissolve better in polar solvents (water, alcohol, ammonia), and non-polar substances in non-polar solvents (benzene, etc.). If the dissolution of a solid in a liquid is accompanied by the absorption of heat, then an increase in temperature leads to an increase in the solubility of the substance.
When solids are dissolved, the volume of the system usually changes insignificantly. Therefore, the solubility of solids in liquids does not depend on pressure.
Henry's law: the mass of a gas that dissolves at a constant temperature in a given volume of liquid is directly proportional to the partial pressure of the gas. C \u003d cr, where C is the mass-volume concentration, p is the partial gas pressure, k is the Henry coefficient.
Consequence of Henry's Law:
a) The volume of a gas dissolved at a constant temperature in a given volume of liquid does not depend on its partial pressure.
b) If there is a mixture of gases above the liquid, then the dissolution of each of them is determined by the partial pressure.
64. Law of distribution. Extraction. Distribution law: a substance that can dissolve in 2 immiscible solvents is distributed between them so that the ratio of its concentrations in these solvents at a constant temperature remains constant, regardless of the total amount of the dissolved substance.
Extraction is a method of extracting a substance from a solution using a suitable solvent (extractant). For extraction from a solution, solvents are used that are immiscible with this solution, but in which the substance dissolves better than in the first solvent.
65. Osmosis. Van't Hoff's law. Osmosis is the one-way diffusion of molecules through a semi-permeable membrane.
When measuring the osmotic pressure of various solutions, it was found that the magnitude of the osmotic pressure depends on the concentration of the solution and on its temperature, but does not depend on the nature of the dissolved substances and the solvent.
P=CRT - van't Hoff's law
where P is the osmotic pressure of the solution (Pa), C is the molarity, R is the universal gas constant, T is the absolute temperature
66. Vapor pressure of solution. Raul's Law. At a given temperature, the vapor pressure over the liquid is a constant value. When a substance is dissolved in a liquid, the vapor pressure of the liquid decreases.
A vapor that is in equilibrium with its liquid is said to be saturated.
Saturated vapor pressure depends on the nature of the liquid and temperature, but does not depend on the volume of the vessel in which the vapor is located.
Thus, the saturation vapor pressure of a solvent over a solution is always lower than over a pure solvent at the same temperature.
The difference between the saturation vapor pressure over the pure solvent and over the solution is called the vapor pressure drop of the solution, and the ratio of the vapor pressure drop of the solution to the saturation vapor pressure over the pure solvent is called the relative vapor pressure drop over the solution.
Raoult's law: the relative decrease in the pressure of the saturated vapor of the solvent over the solution is equal to the mole fraction of the solute.
The phenomenon of a decrease in the pressure of saturated vapor over a solution follows from the Le Chatelier principle.
67. Aqueous solutions of electrolytes. Theory of electrolytic dissociation. a) Dissociation of salts, i.e. crystals with ionic structure.
b) Dissociation upon dissolution of acids, i.e. polar molecules.
Theory of electrolytic dissociation.
Electrolytic dissociation is the process of decomposition of an electrolyte into ions during its dissolution or melting.
The classical theory of electrolytic dissociation was created by S. Arrhenius and W. Ostwald in 1887. Arrhenius adhered to the physical theory of solutions, did not take into account the interaction of an electrolyte with water, and believed that free ions were present in solutions. Russian chemists I. A. Kablukov and V. A. Kistyakovsky used the chemical theory of solutions of D. I. Mendeleev to explain electrolytic dissociation and proved that when an electrolyte is dissolved, it chemically interacts with water, as a result of which the electrolyte dissociates into ions.
The classical theory of electrolytic dissociation is based on the assumption of incomplete dissociation of a solute, characterized by the degree of dissociation α, i.e. fraction of decomposed electrolyte molecules. The dynamic equilibrium between non-dissociated molecules and ions is described by the law of mass action.
68. Strong and weak electrolytes. Degree of dissociation. The degree of dissociation of an electrolyte is the ratio of the number of molecules decomposed into ions in a given solution to the total number of molecules of a given substance in a solution.
Electrolytes, the degree of dissociation of which tends to 1 are called strong: NaCl, NaOH, HCl.
Electrolytes, the degree of dissociation of which tends to 0, are called weak: H 2 O, H 2 CO 2, NH 4 OH.
69. Dissociation constant. Ostwald's dilution law. It is possible to apply the laws that are valid for chemical equilibrium to the equilibria that are established during the dissociation of a weak electrolyte.
The equilibrium constant corresponding to the dissociation of a weak electrolyte is called the dissociation constant.
The value of the equilibrium constant depends on the nature of the electrolyte and solvent, on temperature, but does not depend on the concentration of the solution. This value characterizes the ability of a given acid, base or salt to decompose into ions. The higher the value of the equilibrium constant, the easier the electrolyte dissociates into ions.
Ostwald's law - the degree of dissociation increases as the electrolyte is diluted.
70. The state of strong electrolytes in solution. Activity. Ionic strength. To assess the state of ions in solutions of strong electrolytes, a quantity called activity is used. The activity of an ion is understood as that effective conditional concentration of it, in accordance with which it acts in chemical reactions:
where a is the ion activity, c is the ion concentration, f is the activity coefficient.
For the dilution of solutions, an expression is valid that relates the activity coefficient and the value of the ionic strength of the solution.
lgF= - 0.5Z 2 square root I
If we use activity values, then the laws of chemical equilibrium can also be applied to solutions of strong electrolytes.
71. Properties of acids, bases and salts from the point of view of the theory of electrolytic dissociation. Acids are able to interact with bases. This produces salt and water.
The theory of electrolytic dissociation defines acids as electrolytes that dissociate to form positively charged hydrogen ions.
Similarly, bases are defined as electrolytes that dissociate to form a negatively charged hydroxide ion.
Salts: Hydroxide and hydrogen ions are not formed. They are considered as electrolytes that dissociate to form positively charged ions other than hydrogen ions and negatively charged ions other than hydroxide ion.
Chemical equilibrium is the state of a reversible chemical reaction
aA + b B= c C+ d D,
at which over time there is no change in the concentrations of the reactants in the reaction mixture. The state of chemical equilibrium is characterized chemical equilibrium constant:
where C i are the concentrations of components in equilibrium the perfect mixture.
The equilibrium constant can also be expressed in terms of equilibrium mole fractions X i components:
For reactions occurring in the gas phase, it is convenient to express the equilibrium constant in terms of the equilibrium partial pressures Pi components:
For ideal gases Pi = C i RT and Pi = X i P, where P is the total pressure, so K P, K C and K X are related by the following relation:
K P = K C (RT) c+d–a–b = K X P c+d–a–b. (9.4)
The equilibrium constant is related to r G o chemical reaction:
![]() (9.5)
(9.5)
![]() (9.6)
(9.6)
Change r G or r F in a chemical reaction at given (not necessarily equilibrium) partial pressures Pi or concentrations C i components can be calculated by the equation chemical reaction isotherms (van't Hoff isotherms):
 . (9.7)
. (9.7)
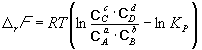 . (9.8)
. (9.8)
According to principle of Le Chatelier If an external influence is exerted on a system in equilibrium, then the equilibrium will shift in such a way as to reduce the effect of external influence. Thus, an increase in pressure shifts the equilibrium in the direction of a decrease in the number of gas molecules. The addition of a reaction component to an equilibrium mixture shifts the equilibrium in the direction of decreasing the amount of this component. An increase (or decrease) in temperature shifts the equilibrium in the direction of a reaction proceeding with the absorption (release) of heat.
Quantitatively, the dependence of the equilibrium constant on temperature is described by the equation isobars of a chemical reaction (van't Hoff isobars)
![]() (9.9)
(9.9)
and isochores of a chemical reaction (van't Hoff isochores)
![]() . (9.10)
. (9.10)
Integration of equation (9.9) under the assumption that r H reaction does not depend on temperature (which is true in narrow temperature ranges), gives:
![]() (9.11)
(9.11)
![]() (9.12)
(9.12)
where C- integration constant. Thus, the dependence ln K P from 1 /T must be linear, and the slope of the straight line is - r H/R.
Integration within K 1 , K 2 , and T 1, T 2 gives:
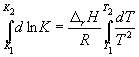 (9.13)
(9.13)
 (9.14)
(9.14)
Using this equation, knowing the equilibrium constants at two different temperatures, we can calculate r H reactions. Accordingly, knowing r H reaction and the equilibrium constant at one temperature, you can calculate the equilibrium constant at another temperature.
EXAMPLES
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500K. f G o for CO(g) and CH 3 OH(g) at 500 K are –155.41 kJ. mol –1 and –134.20 kJ. mol –1, respectively.
Solution. Go reactions:
r G o= f G o(CH 3 OH) - f G o(CO) = –134.20 – (–155.41) = 21.21 kJ. mol -1 .
![]() = 6.09 10 –3 .
= 6.09 10 –3 .
Example 9-2. Reaction equilibrium constant
is equal to K P = 1.64 10 –4 at 400 o C. What total pressure must be applied to an equimolar mixture of N 2 and H 2 to convert 10% N 2 into NH 3 ? The gases are assumed to be ideal.
Solution. Let mol N 2 react. Then
| N 2 (d) | + | 3H 2 (g) | = | 2NH 3 (g) | |
| Initial quantity | 1 | 1 | |||
| Equilibrium quantity | 1– | 1–3 | 2 (Total: 2–2) | ||
| Equilibrium mole fraction: |
Consequently, K X=  and K P = K X . P –2
=
and K P = K X . P –2
= 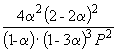 .
.
Substituting = 0.1 into the resulting formula, we have
1.64 10 –4 = , where P= 51.2 atm.
, where P= 51.2 atm.
Example 9-3. Reaction equilibrium constant
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500 K is K P = 6.0910–3. The reaction mixture consisting of 1 mol of CO, 2 mol of H 2 and 1 mol of an inert gas (N 2) is heated to 500 K and a total pressure of 100 atm. Calculate the composition of the equilibrium mixture.
Solution. Let a mole of CO react. Then
| CO(g) | + | 2H 2 (g) | = | CH 3 OH (g) | |
| Initial amount: | 1 | 2 | 0 | ||
| Equilibrium amount: | 1– | 2–2 | |||
| Total in the equilibrium mixture: | 3–2 mol components + 1 mol N 2 \u003d 4–2 mol | ||||
| Equilibrium mole fraction | |||||
Consequently, K X= 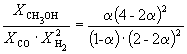 and K P = K X . P-2 =
and K P = K X . P-2 = ![]() .
.
Thus, 6.09 10 –3 = ![]() .
.
Solving this equation, we get = 0.732. Accordingly, the molar fractions of substances in the equilibrium mixture are: = 0.288, = 0.106, = 0.212, and = 0.394.
Example 9-4. For reaction
N 2 (g) + 3H 2 (g) \u003d 2NH 3 (g)
at 298 K K P = 6.0 10 5 , and f H o(NH 3) \u003d -46.1 kJ. mol -1 . Estimate the value of the equilibrium constant at 500 K.
Solution. The standard molar enthalpy of reaction is
r H o= 2f H o(NH 3) \u003d -92.2 kJ. mol -1 .
According to equation (9.14),  =
=
Ln (6.0 10 5) + ![]() = –1.73, whence K 2 =
0.18.
= –1.73, whence K 2 =
0.18.
Note that the equilibrium constant of an exothermic reaction decreases with increasing temperature, which corresponds to Le Chatelier's principle.
TASKS
- At 1273 K and a total pressure of 30 atm in an equilibrium mixture
- At 2000 o C and a total pressure of 1 atm, 2% of water is dissociated into hydrogen and oxygen. Calculate the equilibrium constant of the reaction
- Reaction equilibrium constant
- Reaction equilibrium constant
- A 3-L vessel containing 1.7910–2 mol I 2 was heated to 973 K. The pressure in the vessel at equilibrium turned out to be 0.49 atm. Assuming ideal gases, calculate the equilibrium constant at 973 K for the reaction
- For reaction
- For reaction
- A 1-liter vessel containing 0.341 mol PCl 5 and 0.233 mol N 2 was heated to 250 o C. The total pressure in the vessel at equilibrium was 29.33 atm. Considering all gases to be ideal, calculate the equilibrium constant at 250 o C for the reaction taking place in the vessel
- Reaction equilibrium constant
- At 25°C f G o(NH 3) = –16.5 kJ. mol -1 . Calculate r G reactions of formation of NH 3 at partial pressures of N 2 , H 2 and NH 3 equal to 3 atm, 1 atm and 4 atm, respectively. In which direction will the reaction proceed spontaneously under these conditions?
- exothermic reaction
- The equilibrium constant of the gas-phase isomerization reaction of borneol (C10H17OH) to isoborneol is 0.106 at 503 K. A mixture of 7.5 g of borneol and 14.0 g of isoborneol was placed in a 5-L vessel and kept at 503 K until equilibrium was reached. Calculate the mole fractions and masses of borneol and isoborneol in an equilibrium mixture.
- Equilibrium in reaction
- Calculate the total pressure that must be applied to a mixture of 3 parts H 2 and 1 part N 2 to obtain an equilibrium mixture containing 10% NH 3 by volume at 400 o C. The equilibrium constant for the reaction
- At 250 o C and a total pressure of 1 atm, PCl 5 is dissociated by 80% according to the reaction
- At 2000 o C for the reaction
- Calculate the standard enthalpy of the reaction for which the equilibrium constant is
a) increases by 2 times, b) decreases by 2 times when the temperature changes from 298 K to 308 K. - The dependence of the equilibrium constant of the reaction 2C 3 H 6 (g) \u003d C 2 H 4 (g) + C 4 H 8 (g) on temperature between 300 K and 600 K is described by the equation
CO 2 (g) + C (tv) \u003d 2CO (g)
contains 17% (by volume) CO 2 . What percentage of CO 2 will be contained in the gas at a total pressure of 20 atm? At what pressure will the gas contain 25% CO 2 ?
H 2 O (g) \u003d H 2 (g) + 1 / 2O 2 (g) under these conditions.
CO (g) + H 2 O (g) \u003d CO 2 (g) + H 2 (g)
at 500 o C is Kp= 5.5. A mixture of 1 mol CO and 5 mol H 2 O was heated to this temperature. Calculate the mole fraction of H 2 O in the equilibrium mixture.
N 2 O 4 (g) \u003d 2NO 2 (g)
at 25 o C is Kp= 0.143. Calculate the pressure that will be established in a 1 liter vessel in which 1 g of N 2 O 4 is placed at this temperature.
I 2 (g) = 2I (g).
at 250°C r G o \u003d -2508 J. mol -1. At what total pressure will the degree of conversion of PCl 5 to PCl 3 and Cl 2 at 250 o C be 30%?
2HI (g) \u003d H 2 (g) + I 2 (g)
equilibrium constant K P = 1.83 10 –2 at 698.6 K. How many grams of HI are formed when 10 g of I 2 and 0.2 g of H 2 are heated to this temperature in a three-liter vessel? What are the partial pressures of H 2 , I 2 and HI?
PCl 5 (g) = PCl 3 (g) + Cl 2 (g)
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
at 500 K is K P = 6.0910–3. Calculate the total pressure required to produce methanol with 90% yield if CO and H 2 are taken in a 1:2 ratio.
CO (g) + 2H 2 (g) \u003d CH 3 OH (g)
is in equilibrium at 500 K and 10 bar. If the gases are ideal, how will the following factors affect the yield of methanol: a) increasing T; b) promotion P; c) adding an inert gas at V= const; d) addition of an inert gas at P= const; e) adding H 2 at P= const?
2NOCl (g) \u003d 2NO (g) + Cl 2 (g)
set at 227 o C and a total pressure of 1.0 bar, when the partial pressure of NOCl is equal to 0.64 bar (initially only NOCl was present). Calculate r G o for a reaction. At what total pressure will the partial pressure of Cl 2 be 0.10 bar?
N 2 (g) + 3H 2 (g) \u003d 2NH 3 (g)
at 400 o C is K = 1.60 10 –4 .
PCl 5 (g) = PCl 3 (g) + Cl 2 (g).
What will be the degree of dissociation of PCl 5 if N 2 is added to the system so that the partial pressure of nitrogen is 0.9 atm? The total pressure is maintained at 1 atm.
N 2 (g) + O 2 (g) \u003d 2NO (g)
Kp = 2.510–3. An equilibrium mixture of N 2 , O 2 , NO and an inert gas at a total pressure of 1 bar contains 80% (by volume) N 2 and 16% O 2 . What percentage by volume is NO? What is the partial pressure of an inert gas?
ln K = –1.04 –1088 /T +1.51 10 5 /T 2 .
At a certain temperature, the enthalpy and entropy factors of the reaction can be balanced, then balance state, which corresponds to the equality ∆ r G T= 0. In this state, the free energy of the system is minimal, and the possibility of a direct and reverse reaction is equally probable, while the same amount of reaction products is obtained per unit time as they are consumed in the reverse reaction of the formation of starting materials. Under such conditions, the partial pressures and concentrations of all reaction components will be constant in time and at all points in the system and are called equilibrium pressures and concentrations.
If the reaction proceeds under isochoric-isothermal conditions, then the condition for chemical equilibrium is the equality Δ r F T= 0. It follows from equations (1.12) and (1.15) that at equilibrium of the chemical reaction a A(g)+ b B(g)+ d D(k) ↔ e E(g)+ f F(g)
∆r G 0 T= - RT ln( pe E equals p f F equals / p a A equals pb B equal) . (2.1)
If a given heterogeneous reaction involving gaseous components proceeds at a constant volume, then
∆r F 0 T= - RT ln( c e E equals c f F equals / c a A equals c b B equal) . (2.2)
If the reaction a A(p)+ b B(p)+ d D(k)= e E(p)+ f F(p) proceeds in an ideal solution, then from (1.12a) it follows:
∆r G 0 T=∆r F 0 T = - RT ln( c e E equals c f F equals / c a A equals c b B equal) . (2.3)
Since the quantities ∆ r F 0 T and ∆ r G 0 T for a given temperature there are constant values, then these equations are valid if under the sign of the logarithm there are expressions that are constant for a given temperature, called equilibrium constants K with and K r:
K s = (c e E equals c f F equals / c a A equals c b B equal) (2.4)
K r = (pe E equals p f F equals / p a A equals pb B equal) . (2.5)
Equations (2.4) and (2.5) are the mathematical expression the law of mass action.
For reactions with gaseous components, the relationship between K r and K s is expressed by the equation: K r = K s(RT) ∆ν , (2.6) where ∆ν =( e+f-a-b) is the change in the number of moles of gases as a result of the reaction, and R= 0.082 atm . l . mol -1 . K -1 . It should be noted that in the expression for K s and K r components in a more condensed state are not included (for example, substance D in a crystalline state).
Equilibrium constant K r can also be expressed in terms of the equilibrium amounts of moles of gaseous components n i equal to the total pressure P 0 , at which an isobaric-isothermal reaction is carried out. Given that the partial pressure i-th component is proportional to the mole fraction of this component pi = (n i/Σ n i)P0, from equation (2.5) we obtain:
K r=(pe E equals p f F equals / p a A equals pb B equals)=( ne E equals n f F equals / n a A equals nb B equals)( P0/Σ n i) ∆ν (2.6)
where Σ n i =(n E equals + n F equals + n A equals + n B equal) is the sum of equilibrium moles of all gaseous components.
Combining equations (2.1), (2.2), (2.3) with equations (2.4) and (2.5), we obtain expressions that are often used for calculations:
∆r G 0 T= - RT ln K r and (2.7)
∆r F 0 T= - RT ln K s for gas-phase reactions. (2.8)
∆r G 0 T =- RT ln K s for reactions in condensed systems. (2.7a)
Thus, having calculated the Gibbs energy of the reaction for a given temperature, we can use these formulas to calculate K s and K r at this temperature. The greater the value of the equilibrium constant under these conditions, the greater the values of the equilibrium concentrations of the reaction products, therefore, the higher the yield of the reaction products. The yield of a reaction product is understood as the ratio of the amount (or mass) of the reaction product that was formed under given conditions to the maximum possible (theoretically) amount (or mass) of this product, provided that any initial substance is completely converted into the reaction product. It is obvious that the complete (100%) transformation of the starting substance into a product is impossible from the thermodynamic point of view, since in this case the equilibrium constant becomes infinitely large.
Under the degree of conversion of the original substance understand the ratio of the amount (or mass) of the original substance, which reacted under given conditions, to the initial amount (or mass) of this substance. If the yield of the product tends to unity (100%), then the degree of conversion of the starting material also approaches unity (100%).
Values K r and K s at a given temperature do not depend on the values of partial pressures and concentrations of components, as well as the total pressure in the system, but depend on temperature. The dependence of the equilibrium constant on temperature can be expressed in differential form:
(d ln Kp/ dT) = ∆r H 0/(RT 2) , (2.9) where ∆ r N 0 is the standard enthalpy of reaction, which is assumed to be independent of temperature as a first approximation. As can be seen from (2.9), with increasing temperature, the equilibrium constant of the exothermic reaction decreases, and the equilibrium constant of the endothermic reaction increases.
When integrating expression (2.9), taking into account the indicated approximation, we obtain (for T 2 > T 1) formula
ln( K 2 /K 1) = (∆r H 0/R)(1/T 1 – 1/T 2) , (2.10)
from which it follows that the greater the absolute value of the thermal effect of the reaction, the more the value of the equilibrium constant changes with temperature. This formula can also be used to calculate the value K equals at any T 3 if values are known To 2 and To 1 at temperatures T 2 and T 1 .
Example 10 Write an expression for K s and K r and calculate K r and K s reactions C (c) + CO 2 (g) \u003d 2CO (g) at 298 K and at 1000 K. Draw a conclusion from the obtained values about the yield of the reaction product at given temperatures and about the effect of temperature on the value of the equilibrium constant.
Solution. Let us write down the expressions for the equilibrium constants of this reaction, taking into account that the reaction is heterogeneous and the graphite C(k) substance is in the solid state:
K p = p 2 CO equals / p CO 2 equal; K s = With 2 CO equals / With CO 2 equal
From equation (2.7) we have Kp=exp(- ∆G 0 T/RT). Using the results of Example 5, we calculate K r for 298 K and 1000 K:
K r 298 = exp(-120 . 10 3 /8,31 . 298)= exp(-48.5)<< 1;
Kp 1000 =exp(+316/8.31 . 1000)= exp(0.038) = 1.039.
By formula (2.6) we find K s = K r/(RT) ∆ν = 1.039/0.082 . 1000 = 0.013 since ∆ν = 2-1=1. Based on the data obtained, it can be concluded that at 298 K the equilibrium constant K r tends to zero, which indicates that there are practically no reaction products in the equilibrium mixture and the reaction equilibrium is strongly shifted towards the starting materials. With increasing temperature, the value of the equilibrium constant increases (the reaction is endothermic) and at 1000 K K r is already greater than 1, that is, the reaction products begin to predominate in the equilibrium mixture, their yield increases with increasing T.
Example 11. For some reaction A(r) = 2B(r) proceeding at constant pressure and temperature, the equilibrium constant K r is 0.02 at 400 K and 4.0 at 600 K. From these data determine ∆ r H 0 298 , ∆r S 0 298 and ∆ r G 0 298 this reaction, and K r at 800 K.
Solution. Neglecting the dependence ∆ r H 0 and ∆ r S 0 on temperature and using expressions (1.14) and (2.7) we compose a system of two equations with two unknowns ( T 1 =400K, T 2=600K):
∆r G 0 T 1 =∆r H 0 298 –T 1 ∆r S 0 298= -RT 1ln K r 1 or x – 400y= -8.31.400 ln2 . 10-2
∆r G 0 T 2 =∆r H 0 298 –T 2 ∆r S 0 298= -RT 2ln K r 2 or x – 600y= -8.31 . 600ln4
Where X = ∆r H 0 298 \u003d 52833 (J) \u003d 52.833 kJ; y =∆r S 0 298 \u003d 99.575 J / K.
Meaning K r at 800 K we calculate by formula (2.10). We have:
ln( K 800 /K 400) = log( K 800 / 0.02) = (52833 / 8.31) (1/400 -1/800) = 7.95. Where To 800 = 56,55.
Example 10 Determine the temperature at which in the reaction CaCO 3 (k) \u003d CaO (c) + CO 2 (g) the equilibrium partial pressure of CO 2 R CO2 = 10 4 Pa.
Solution. For this heterogeneous reaction, we write the expression for the equilibrium constant: K r = R CO2, that is, the equilibrium constant is equal to the relative partial pressure of CO 2 at a given temperature. For desired temperature K r =R CO2 \u003d 10 4 /10 5 \u003d 0.1. Neglecting the dependence ∆ r H 0 and ∆ r S 0 on temperature, we use formulas (1.14) and (2.7) and equate two expressions for ∆ r G 0 T : ∆r G 0 T= ∆r H 0 298 –T∆r S 0 298= -RT ln K r. ∆ values r H 0 298 and ∆ r S 0 298 is determined, as discussed above, according to tabular data: ∆r H 0 298 = 178.1 kJ; ∆r S 0 298 \u003d 160.5 J. We have:
178,1 . 10 3 –T . 160,5
∆r G 0 T= -8,31T ln0.1
Solving the resulting system of equations with respect to T, find T=991K
Chemical equilibrium constant- a characteristic of a chemical reaction, by the value of which one can judge the direction of the process at the initial ratio of the concentrations of the reactants, the maximum possible yield of the reaction product under certain conditions.
The chemical equilibrium constant is determined by the law of mass action. Its values are calculated or based on experimental data. The chemical equilibrium constant depends on the nature of the reactants and on the temperature.
Equilibrium constant and Gibbs energy
The equilibrium constant ~K is related to the Gibbs free energy ~\Delta G as follows:
~\Delta G=-RT\cdot\ln K.The above equation makes it possible to calculate K from the value of ΔG°, and then the equilibrium concentrations (partial pressures) of the reagents.
It can be seen from this equation that the equilibrium constant is very sensitive to changes in temperature (if we express the constant from here, then the temperature will be in the exponent). For endothermic processes, an increase in temperature corresponds to an increase in the equilibrium constant, for exothermic processes, to its decrease. The equilibrium constant does not depend on pressure, except for cases of very high pressure (from 100 Pa).
The dependence of the equilibrium constant on the enthalpy and entropy factors indicates the influence of the nature of the reagents on it.
Equilibrium constant and reaction rate
You can express the equilibrium constant in terms of the reaction rate. In this case, the equilibrium constant is defined as
~K=\frac(k_1)(k_(-1)),where ~k_1 is the rate constant of the forward reaction, ~k_(-1) is the rate constant of the reverse reaction.
A quantitative characteristic showing the direction of the reaction and the shift in the concentration of substances is called the equilibrium constant of a chemical reaction. The equilibrium constant depends on the temperature and the nature of the reactants.
Reversible and irreversible reactions
All reactions can be divided into two types:
- reversible, simultaneously flowing in two mutually opposite directions;
- irreversible flowing in the same direction with the total consumption of at least one initial substance.
In irreversible reactions, insoluble substances are usually formed in the form of a precipitate or gas. These reactions include:
- combustion:
C 2 H 5 OH + 3O 2 → 2CO 2 + H 2 O;
- decomposition:
2KMnO 4 → K 2 MnO 4 + MnO 2 + H 2 O;
- connection with the formation of a precipitate or gas:
BaCl 2 + Na 2 SO 4 → BaSO 4 ↓ + 2NaCl.

Rice. 1. Precipitation of BaSO 4 .
Reversible reactions are possible only under certain constant conditions. The original substances give a new substance, which immediately breaks down into its constituent parts and is collected again. For example, as a result of the reaction 2NO + O 2 ↔ 2NO 2 nitric oxide (IV) easily decomposes into nitric oxide (II) and oxygen.
Equilibrium
After a certain time, the rate of the reversible reaction slows down. Chemical equilibrium is achieved - a state in which there is no change in the concentration of the starting substances and reaction products over time, since the rates of the forward and reverse reactions are equalized. Equilibrium is possible only in homogeneous systems, that is, all reacting substances are either liquids or gases.
Consider the chemical equilibrium on the example of the reaction of the interaction of hydrogen with iodine:
- direct reaction -
H 2 + I 2 ↔ 2HI;
- back reaction -
2HI ↔ H 2 + I 2 .
As soon as two reagents are mixed - hydrogen and iodine - hydrogen iodine does not yet exist, since simple substances only react. A large number of starting substances actively react with each other, so the rate of the direct reaction will be maximum. In this case, the reverse reaction does not proceed, and its rate is zero.
The rate of a direct reaction can be expressed graphically:
ν pr = k pr ∙ ∙ ,
where k pr is the rate constant of the direct reaction.
Over time, the reagents are consumed, their concentration decreases. Accordingly, the rate of the forward reaction decreases. At the same time, the concentration of a new substance, hydrogen iodide, increases. When accumulated, it begins to decompose, and the rate of the reverse reaction increases. It can be expressed as
ν arr = k arr ∙ 2 .
Hydrogen iodide is squared, since the coefficient of the molecule is two.
At some point, the rates of the forward and reverse reactions equalize. There is a state of chemical equilibrium.

Rice. 2. Graph of reaction rate versus time.
The equilibrium can be shifted either towards the starting materials or towards the products of the reaction. The displacement under the influence of external factors is called Le Chatelier's principle. Equilibrium is affected by temperature, pressure, concentration of one of the substances.
Constant calculation
In a state of equilibrium, both reactions proceed, but at the same time, the concentrations of substances are in equilibrium (equilibrium concentrations are formed), since the rates are balanced (ν pr \u003d ν arr).
Chemical equilibrium is characterized by the chemical equilibrium constant, which is expressed by the summary formula:
K p \u003d k pr / k arr \u003d const.
The reaction rate constants can be expressed in terms of the reaction rate ratio. Let's take the conditional equation of the reverse reaction:
aA + bB ↔ cC + dD.
Then the rates of the forward and reverse reactions will be equal:
- ν inc = k inc ∙ [A] p a ∙ [B] p b
- ν arr = k arr ∙ [C] p c ∙ [D] p d .
Accordingly, if
ν pr \u003d ν arr,
k ex ∙ [A] p a ∙ [B] p b = k arr ∙ [C] p c ∙ [D] p d .
From here we can express the ratio of constants:
k arr / k inc = [C] p c ∙ [D] p d / [A] p a ∙ [B] p b .
This ratio is equal to the equilibrium constant:
K p = [C] p c ∙ [D] p d / [A] p a ∙ [B] p b .

Rice. 3. The formula for the equilibrium constant.
The value shows how many times the rate of the forward reaction is greater than the rate of the reverse reaction.
What have we learned?
Reactions depending on the final products are classified into reversible and irreversible. Reversible reactions proceed in both directions: the starting materials form final products, which decompose into starting substances. During a reaction, the rates of the forward and reverse reactions are balanced. This state is called chemical equilibrium. It can be expressed as the ratio of the product of the equilibrium concentrations of the reaction products to the product of the equilibrium concentrations of the starting materials.
Topic quiz
Report Evaluation
Average rating: 4.8. Total ratings received: 193.
