Magnetic permeability. Magnetic properties of substances Relative magnetic permeability of a substance
From many years of technical practice, we know that the inductance of a coil is highly dependent on the characteristics of the environment where this coil is located. If a ferromagnetic core is added to a copper wire coil with a known inductance L0, then under other previous circumstances, the self-induction currents (extra currents of closing and opening) in this coil will increase many times, the experiment will confirm this, which will mean that it has increased several times, which now becomes equal to L.
Experimental observation
Let us assume that the environment, the substance that fills the space inside and around the described coil, is homogeneous, and generated by the current flowing through its wire, is localized only in this designated area, without going beyond its boundaries.
If the coil has a toroidal shape, the shape of a closed ring, then this medium, together with the field, will be concentrated only inside the volume of the coil, since there is almost no magnetic field outside the toroid. This position is also valid for a long coil - a solenoid, in which all magnetic lines are also concentrated inside - along the axis.
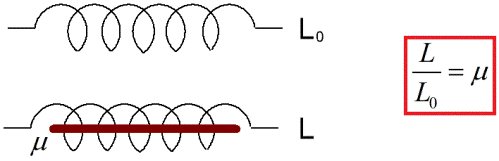
For example, let's assume that the inductance of some circuit or coil without a core in vacuum is L0. Then for the same coil, but already in a homogeneous substance that fills the space where the magnetic field lines of this coil are present, let the inductance be equal to L. In this case, it turns out that the ratio L / L0 is nothing more than the relative magnetic the permeability of the named substance (sometimes simply called "magnetic permeability").
It becomes obvious: magnetic permeability is a value that characterizes the magnetic properties of a given substance. It often depends on the state of the substance (and on environmental conditions such as temperature and pressure) and on its kind.
Understanding the term

The introduction of the term "magnetic permeability", in relation to a substance placed in a magnetic field, is similar to the introduction of the term "dielectric constant" for a substance located in an electric field.
The value of the magnetic permeability, determined by the above formula L/L0, can also be expressed as the ratio of the absolute magnetic permeability of a given substance and absolute emptiness (vacuum).
It is easy to see: relative magnetic permeability (it is also magnetic permeability) is a dimensionless quantity. But the absolute magnetic permeability - has the dimension of Gn / m, the same as that of the magnetic permeability (absolute!) of vacuum (it is also the magnetic constant).
![]()
In fact, we see that the medium (magnet) affects the inductance of the circuit, and this clearly indicates that a change in the medium leads to a change in the magnetic flux Ф penetrating the circuit, and hence to a change in the induction B, in relation to any point of the magnetic field.
The physical meaning of this observation is that with the same coil current (with the same magnetic intensity H), the induction of its magnetic field will be a certain number of times greater (in some cases less) in a substance with a magnetic permeability mu than in full vacuum.
This is because, and itself begins to have a magnetic field. Substances that can be magnetized in this way are called magnets.
The unit of measurement of absolute magnetic permeability is 1 Gn / m (henry per meter or newton per ampere squared), that is, it is the magnetic permeability of such a medium, where, at a magnetic field strength H equal to 1 A / m, a magnetic induction of 1 T occurs.
Physical picture of the phenomenon
From the foregoing, it becomes clear that various substances (magnets) are magnetized under the influence of the magnetic field of the circuit with current, and as a result, a magnetic field is obtained, which is the sum of magnetic fields - the magnetic field from the magnetized medium plus from the circuit with current, therefore it differs in magnitude from the field only circuits with current without medium. The reason for the magnetization of magnets lies in the existence of the smallest currents inside each of their atoms.

According to the value of magnetic permeability, substances are classified into diamagnets (less than one - they are magnetized against the applied field), paramagnets (more than one - they are magnetized in the direction of the applied field) and ferromagnets (much more than one - they are magnetized, and have magnetization after turning off the applied magnetic field).
It is characteristic of ferromagnets, therefore the concept of "magnetic permeability" in its pure form is not applicable to ferromagnets, but in a certain range of magnetization, in some approximation, it is possible to single out a linear section of the magnetization curve, for which it will be possible to estimate the magnetic permeability.
Superconductors have a magnetic permeability of 0 (because the magnetic field is completely displaced from their volume), and the absolute magnetic permeability of air is almost equal to the vacuum mu (read the magnetic constant). For air, mu is slightly more than 1.
Magnetic permeability is different for different media and depends on its properties, therefore it is customary to talk about the magnetic permeability of a particular medium (meaning its composition, state, temperature, etc.).
In the case of a homogeneous isotropic medium, the magnetic permeability μ:
μ \u003d B / (μ o H),
In anisotropic crystals, the magnetic permeability is a tensor.
Most substances are divided into three classes according to the value of magnetic permeability:
- diamagnets ( μ < 1 ),
- paramagnets ( µ > 1 )
- ferromagnets (having more pronounced magnetic properties, such as iron).
The magnetic permeability of superconductors is zero.
The absolute magnetic permeability of air is approximately equal to the magnetic permeability of vacuum and in technical calculations is taken equal to 4π 10 -7 H/m
μ = 1 + χ (in SI units);
μ = 1 + 4πχ (in CGS units).
The magnetic permeability of the physical vacuum μ =1, since χ=0.
Magnetic permeability shows how many times the absolute magnetic permeability of a given material is greater than the magnetic constant, i.e., how many times the magnetic field of macrocurrents H is enhanced by the field of microcurrents of the medium. The magnetic permeability of air and most substances, with the exception of ferromagnetic materials, is close to unity.
Several types of magnetic permeability are used in the technique, depending on the specific applications of the magnetic material. Relative magnetic permeability shows how many times in a given medium the force of interaction between wires with current changes compared to vacuum. Numerically equal to the ratio of the absolute magnetic permeability to the magnetic constant. The absolute magnetic permeability is equal to the product of the magnetic permeability and the magnetic constant.
For diamagnets, χμχ>0 and μ> 1. Depending on whether μ of ferromagnets is measured in a static or alternating magnetic field, it is called, respectively, static or dynamic magnetic permeability.
The magnetic permeability of ferromagnets depends in a complex way on H . From the magnetization curve of a ferromagnet, one can construct the dependence of the magnetic permeability on N.
Magnetic permeability, determined by the formula:
μ \u003d B / (μ o H),
called static magnetic permeability.
It is proportional to the tangent of the slope of the secant drawn from the origin through the corresponding point on the main magnetization curve. The limiting value of the magnetic permeability μ n with a magnetic field tending to zero is called the initial magnetic permeability. This characteristic is of great importance in the technical use of many magnetic materials. Experimentally, it is determined in weak magnetic fields with a strength of the order of 0.1 A/m.
Dielectric constant of substances
Substance |
Substance |
||
Gases and water vapor |
Liquids |
||
| Nitrogen | 1,0058 | Glycerol | 43 |
| Hydrogen | 1,00026 | Liquid oxygen (at t = -192.4 o C) | 1,5 |
| Air | 1,00057 | Transformer oil | 2,2 |
| Vacuum | 1,00000 | Alcohol | 26 |
| Water vapor (at t=100 o C) | 1,006 | Ether | 4,3 |
| Helium | 1,00007 | Solids |
|
| Oxygen | 1,00055 | Diamond | 5,7 |
| Carbon dioxide | 1,00099 | Waxed paper | 2,2 |
Liquids |
wood dry | 2,2-3,7 | |
| Liquid nitrogen (at t = -198.4 o C) | 1,4 | Ice (at t = -10 o C) | 70 |
| Petrol | 1,9-2,0 | Paraffin | 1,9-2,2 |
| Water | 81 | Rubber | 3,0-6,0 |
| Hydrogen (at t= - 252.9 o C) | 1,2 | Mica | 5,7-7,2 |
| Helium liquid (at t = - 269 o C) | 1,05 | Glass | 6,0-10,0 |
| barium titanate | 1200 | ||
| Porcelain | 4,4-6,8 | ||
| Amber | 2,8 | ||
Note. Electrical constant ԑ o (vacuum permittivity) equal to: ԑ o = 1\4πs 2 * 10 7 F / m ≈ 8.85 * 10 -12 F / m
Magnetic permeability of a substance
Note. Magnetic constant μ o (vacuum magnetic permeability) is: μ o = 4π * 10 -7 H/m ≈ 1.257 * 10 -6 H/m
Magnetic permeability of ferromagnets
The table shows the values of magnetic permeability for some ferromagnets (substances with μ > 1). Magnetic permeability for ferromagnets (iron, cast iron, steel, nickel, etc.) is not constant. The table shows the maximum values.
1 Permalloy-68- an alloy of 68% nickel and 325 iron; This alloy is used to make transformer cores.
Curie temperature
Electrical resistivity of materials
High resistance alloys
Alloy name |
Electrical resistivity µOhm m |
Alloy composition, % |
|||
Manganese |
Other elements |
||||
| Constantan | 0,50 | 54 | 45 | 1 | - |
| Kopel | 0,47 | 56,5 | 43 | 0,05 | - |
| Manganin | 0,43 | > 85 | 2-4 | 12 | - |
| Nickel silver | 0,3 | 65 | 15 | - | 20 Zn |
| Nickelin | 0,4 | 68,5 | 30 | 1,5 | - |
| Nichrome | 1,1 | - | > 60 | < 4 | 30 < Cr ост. Fe |
| Fechral | 1,3 | - | - | - | 12-15 Cr 3-4 Al 80< Fe |
Temperature coefficients of electrical resistance of conductors
Conductor |
Conductor |
||
| Aluminum | Nickel | ||
| Tungsten | Nichrome | ||
| Iron | Tin | ||
| Gold | Platinum | ||
| Constantan | Mercury | ||
| Brass | Lead | ||
| Magnesium | Silver | ||
| Manganin | Steel | ||
| Copper | Fechral | ||
| Nickel silver | Zinc | ||
| Nickelin | Cast iron |
Superconductivity of conductors
- Notes.
- Superconductivity found in more than 25 metallic elements and in a large number of alloys and compounds.
- The superconductor with the highest transition temperature to the superconducting state -23.2 K (-250.0 o C) - until recently was niobium germanide (Nb 3 Ge). At the end of 1986, a superconductor with a transition temperature of ≈ 30 K (≈ -243 o C) was obtained. The synthesis of new high-temperature superconductors is reported: ceramics (produced by sintering barium, copper and lanthanum oxides) with a transition temperature of ≈ 90-120 K.
Electrical resistivity of some semiconductors and dielectrics
| Substance | GlassTemperature, o С | Resistivity | |
| Ohm m | Ohm mm2/m | ||
Semiconductors |
|||
| Antimonide indium | 17 | 5.8 x 10 -5 | 58 |
| Bor | 27 | 1.7 x 10 4 | 1.7 x 10 10 |
| Germanium | 27 | 0,47 | 4.7 x 10 5 |
| Silicon | 27 | 2.3 x 10 3 | 2.3 x 10 9 |
| Lead (II) selenide (PbSe) | 20 | 9.1 x 10 -6 | 9,1 |
| Lead(II) sulfide (PbS) | 20 | 1.7 x 10 -5 | 0,17 |
Dielectrics |
|||
| Distilled water | 20 | 10 3 -10 4 | 10 9 -10 10 |
| Air | 0 | 10 15 -10 18 | 10 21 -10 24 |
| Beeswax | 20 | 10 13 | 10 19 |
| Dry wood | 20 | 10 9 -10 10 | 10 15 -10 16 |
| Quartz | 230 | 10 9 | 10 15 |
| Transformer oil | 20 | 10 11 -10 13 | 10 16 -10 19 |
| Paraffin | 20 | 10 14 | 10 20 |
| Rubber | 20 | 10 11 -10 12 | 10 17 -10 18 |
| Mica | 20 | 10 11 -10 15 | 10 17 -10 21 |
| Glass | 20 | 10 9 -10 13 | 10 15 -10 19 |
Electrical properties of plastics
| plastic name | The dielectric constant | |
| Getinax | 4,5-8,0 | 10 9 -10 12 |
| Kapron | 3,6-5,0 | 10 10 -10 11 |
| Lavsan | 3,0-3,5 | 10 14 -10 16 |
| Organic glass | 3,5-3,9 | 10 11 -10 13 |
| Styrofoam | 1,0-1,3 | ≈ 10 11 |
| Polystyrene | 2,4-2,6 | 10 13 -10 15 |
| PVC | 3,2-4,0 | 10 10 -10 12 |
| Polyethylene | 2,2-2,4 | ≈ 10 15 |
| Fiberglass | 4,0-5,5 | 10 11 -10 12 |
| Textolite | 6,0-8,0 | 10 7 -10 19 |
| Celluloid | 4,1 | 10 9 |
| Ebonite | 2,7-3,5 | 10 12 -10 14 |
Electrical resistivity of electrolytes (at t=18 o C and 10% solution concentration)
Note. The specific resistance of electrolytes depends on temperature and concentration, i.e. from the ratio of the mass of dissolved acid, alkali or salt to the mass of dissolving water. At the indicated concentration of solutions, an increase in temperature by 1 o C reduces the resistivity of a solution taken at 18 o C by 0.012 sodium hydroxide, by 0.022 - for copper sulfate, by 0.021 - for sodium chloride, by 0.013 - for sulfuric acid and by 0.003 - for 100% sulfuric acid.
Specific electrical resistance of liquids
Liquid |
Specific electrical resistance, Ohm m |
Liquid |
Specific electrical resistance, Ohm m |
| Acetone | 8.3 x 10 4 | Molten Salts: | |
| distilled water | 10 3 - 10 4 | potassium hydroxide (KOH; at t = 450 o C) | 3.6 x 10 -3 |
| sea water | 0,3 | sodium hydroxide (NaOH; at t = 320 o C) | 4.8 x 10 -3 |
| river water | 10-100 | sodium chloride (NaCI; at t = 900 o C) | 2.6 x 10 -3 |
| Liquid air (at t = -196 o C) | 10 16 | soda (Na 2 CO 3 x10H 2 O; at t = 900 o C) | 4.5 x 10 -3 |
| Glycerol | 1.6 x 10 5 | Alcohol | 1.5 x 10 5 |
| Kerosene | 10 10 | ||
| Melted naphthalene (at (at t = 82 o C) | 2.5 x 10 7 | ||
Magnetic properties of substances
Just as the electrical properties of a substance are characterized by the permittivity, the magnetic properties of a substance are characterized by magnetic permeability.
Due to the fact that all substances in a magnetic field create their own magnetic field, the magnetic induction vector in a homogeneous medium differs from the vector at the same point in space in the absence of a medium, i.e., in vacuum.
The relation is called magnetic permeability of the medium.
So, in a homogeneous medium, the magnetic induction is equal to:
The value of m for iron is very large. This can be verified by experience. If an iron core is inserted into a long coil, then the magnetic induction, according to formula (12.1), will increase m times. Consequently, the flux of magnetic induction will increase by the same amount. When the circuit that feeds the magnetizing coil with direct current is opened, an induction current appears in the second, small coil wound over the main one, which is recorded by a galvanometer (Fig. 12.1).
If an iron core is inserted into the coil, then the deviation of the galvanometer needle when the circuit is opened will be m times greater. Measurements show that the magnetic flux when an iron core is introduced into the coil can increase thousands of times. Therefore, the magnetic permeability of iron is enormous.

There are three main classes of substances with sharply different magnetic properties: ferromagnets, paramagnets and diamagnets.
ferromagnets
Substances in which, like iron, m >> 1, are called ferromagnets. In addition to iron, cobalt and nickel, as well as a number of rare earth elements and many alloys, are ferromagnets. The most important property of ferromagnets is the existence of residual magnetism. A ferromagnetic substance can be in a magnetized state without an external magnetizing field.
An iron object (for example, a rod) is known to be drawn into a magnetic field, that is, it moves to an area where the magnetic induction is greater. Accordingly, it is attracted to a magnet or an electromagnet. This happens because the elementary currents in iron are oriented in such a way that the direction of the magnetic induction of their field coincides with the direction of the induction of the magnetizing field. As a result, the iron rod turns into a magnet, the nearest pole of which is opposite to the pole of the electromagnet. Opposite poles of magnets are attracted (Fig. 12.2).
Rice. 12.2 
STOP! Decide for yourself: A1-A3, B1, B3.
Paramagnets
There are substances that behave like iron, that is, they are drawn into a magnetic field. These substances are called paramagnetic. These include some metals (aluminum, sodium, potassium, manganese, platinum, etc.), oxygen and many other elements, as well as various electrolyte solutions.
Since paramagnets are drawn into the field, the lines of induction of their own magnetic field created by them and the magnetizing field are directed in the same direction, so the field is amplified. Thus, they have m > 1. But m differs from unity very slightly, only by a value of the order of 10 -5 ... 10 -6 . Therefore, powerful magnetic fields are required to observe paramagnetic phenomena.
Diamagnets
A special class of substances are diamagnets discovered by Faraday. They are pushed out of the magnetic field. If you hang a diamagnetic rod near the pole of a strong electromagnet, then it will repel from it. Consequently, the lines of induction of the field created by him are directed opposite to the lines of induction of the magnetizing field, that is, the field is weakened (Fig. 12.3). Accordingly, for diamagnets m< 1, причем отличается от единицы на величину порядка 10 –6 . Магнитные свойства у диамагнетиков выражены слабее, чем у парамагнетиков.
 Rice. 12.3
Rice. 12.3
 Rice. 12.4 Rice. 12.4 |
Diamagnets include bismuth, copper, sulfur, mercury, chlorine, inert gases, and almost all organic compounds. Diamagnetic is a flame, such as a candle flame (mainly due to carbon dioxide). Therefore, the flame is pushed out of the magnetic field (Fig. 12.4) .
The magnetic field of the coil is determined by the current and the intensity of this field, and the field induction. Those. the field induction in vacuum is proportional to the magnitude of the current. If a magnetic field is created in a certain medium or substance, then the field acts on the substance, and it, in turn, changes the magnetic field in a certain way.
A substance in an external magnetic field becomes magnetized and an additional internal magnetic field arises in it. It is associated with the movement of electrons along intraatomic orbits, as well as around their own axis. The motion of electrons and nuclei of atoms can be considered as elementary circular currents.
The magnetic properties of an elementary circular current are characterized by a magnetic moment.
In the absence of an external magnetic field, the elementary currents inside the substance are oriented randomly (chaotically) and, therefore, the total or total magnetic moment is zero and the magnetic field of elementary internal currents is not detected in the surrounding space.
The effect of an external magnetic field on elementary currents in matter is that the orientation of the axes of rotation of charged particles changes so that their magnetic moments turn out to be directed in one direction. (toward the external magnetic field). The intensity and nature of magnetization in different substances in the same external magnetic field differ significantly. The value characterizing the properties of the medium and the influence of the medium on the magnetic field density is called absolute magnetic permeability or magnetic permeability of the medium (μ With ) . This is the relation = . Measured [ μ With ]=H/m.
The absolute magnetic permeability of vacuum is called the magnetic constant μ about \u003d 4π 10 -7 Gn / m.
The ratio of the absolute magnetic permeability to the magnetic constant is called relative magnetic permeabilityμ c /μ 0 \u003d μ. Those. relative magnetic permeability is a value showing how many times the absolute magnetic permeability of a medium is greater or less than the absolute permeability of vacuum. μ is a dimensionless quantity that varies over a wide range. This value is the basis for dividing all materials and media into three groups.
Diamagnets . These substances have μ< 1. К ним относятся - медь, серебро, цинк, ртуть, свинец, сера, хлор, вода и др. Например, у меди μ Cu = 0,999995. Эти вещества слабо взаимодействуют с магнитом.
Paramagnets . These substances have μ > 1. These include aluminum, magnesium, tin, platinum, manganese, oxygen, air, etc. Air has = 1.0000031. . These substances, as well as diamagnets, weakly interact with a magnet.
For technical calculations, μ of diamagnetic and paramagnetic bodies is assumed to be equal to one.
ferromagnets . This is a special group of substances that play a huge role in electrical engineering. These substances have μ >> 1. These include iron, steel, cast iron, nickel, cobalt, gadolinium and metal alloys. These substances are strongly attracted to a magnet. These substances have μ = 600-10,000. For some alloys, μ reaches record values up to 100,000. It should be noted that μ for ferromagnetic materials is not constant and depends on the magnetic field strength, type of material and temperature.
The large value of µ in ferromagnets is explained by the fact that they have regions of spontaneous magnetization (domains), within which the elementary magnetic moments are directed in the same way. When added together, they form the common magnetic moments of the domains.
In the absence of a magnetic field, the magnetic moments of the domains are randomly oriented and the total magnetic moment of the body or substance is zero. Under the action of an external field, the magnetic moments of the domains are oriented in one direction and form the total magnetic moment of the body, directed in the same direction as the external magnetic field.
This important feature is used in practice, using ferromagnetic cores in coils, which makes it possible to sharply increase the magnetic induction and magnetic flux at the same values of currents and the number of turns, or, in other words, to concentrate the magnetic field in a relatively small volume.
