Depends on pH. Hydrogen index (pH factor)
In this article, we answer the questions of what is the acidity of wine and how it is determined. What is pH and why should the consumer know it. What is a degree of alcohol.
degree of alcohol
One of these abbreviations is very simple - ABV means the English "alcohol by volume", those. the alcohol content (in our case, ethanol) in the liquid volume. Usually measured as a percentage. And in colloquial speech it is called a degree. For example, the expression forty-degree vodka means that the proposed solution contains 40% - forty percent alcohol by volume.
Volume percentage or degree is measured in milliliters of "pure" ethanol in a volume of 100 ml at a temperature of 20 degrees Celsius.
In a nutshell, it is clear that if the bottle indicates ABV 5.5%, as, for example, on some Moscato d’Asti wines, then this low-carbonated and low-alcohol wine can be lightly sipped all evening without fear of getting a hangover the next day. As they say, there is more alcohol in kefir!
By the way, this is why Moscato d'Asti and another Italian sparkling wine, Prosecco, are so popular at Hollywood parties. Everyone walks all evening with a glass in hand, but there are no drunks. And you can drive home yourself. Although judging by the news, the participants of these parties do not really care about the latter consideration.
A bit of theory - what is pH
On an intuitive level, we all roughly understand what acidity is. The degree of "acidity", so to speak. In chemistry, this term is acidity, lat. aciditas, eng. acidity - denotes a characteristic of the activity of hydrogen ions in solutions and liquids.
There are true (active) and total (titratable) acidity. In aqueous solutions, inorganic substances, i.e. salts, acids and alkalis (dissolved) are separated into their constituent ions.
At the same time, positively charged hydrogen ions H+ are carriers of acidic properties, and negatively charged ions OH-(they are also called hydroxyls) - carriers of alkaline properties.
A hundred years ago, chemists introduced a special hydrogen index, which is usually denoted by the symbols pH.
A bit of math
Non-nudists(c) and non-mathematicians(c) may skip this paragraph. And for the rest, we will inform you that for aqueous solutions, the equilibrium equation applies - the product of the activity of H + and OH- ions is constant. Under so-called normal conditions, ie. at a water temperature of 22°C and normal pressure, it is equal to 10 to the minus 14th power.
The Danish biochemist Sorensen in 1909 introduced the pH value, which by definition is equal to the decimal logarithm of the activity of hydrogen ions, taken with a minus:
pH= - lg (H+ activity)
In a neutral medium, as we have just said, the activities of the ions are equal, i.e. the product of H+ activity and OH- activity is equal to the square of H+ activity. And it is equal to 10 to the minus 14th power.
So, after dividing 14 by 2, the negative decimal logarithm will be equal to 7. This means that (at a temperature of 22 ° C) the acidity of pure water, that is, neutral acidity, is equal to seven units: pH= 7.
Solutions and liquids are considered acidic if they pH less than 7, and alkaline, if more.
Typically, food products, including wine, tend to be acidic. Alkaline reactions are chemical dough leavening agents (soda, ammonium carbonate) and products prepared with their use, such as cookies and gingerbread.
Three types of acidity
Let's get back to guilt. The term "acidity" is one of the most used in the analysis, description and production of wines. In fact, acidity is one of the most important characteristics of wine chemistry and taste. There are three types of acidity in winemaking:
- total or titrated
- active or true - this is the [hydrogen] indicator of activity pH
- volatile acidity
Titratable acidity
Titratable or total acidity determines the content in the juice or wine of all free acids and their acid salts in the aggregate.
Its value is determined by the amount of alkali (for example, caustic soda or potassium) needed to neutralize these acids. That is, the amount of alkali that must be added to wine in order to obtain an absolutely neutral solution from it (pH=7.0).
Total acidity is measured in grams per litre.
Active acidity
Active or true acidity pH . Mathematically, this is the negative logarithm of the concentration of hydrogen ions, as mentioned above. Technically, this is the most accurate measure of wine acidity.
It depends on the amount of the strongest acids contained in the wine. Strong acids are those that have the highest dissociation constant (Kd) [acids].
An example of typical acids ordered by "strength", that is, in descending order of the dissociation constant (degree of acid):
- Lemon Cd = 8.4 10-4
- Amber Cd = 7.4 10-4
- Apple Cd = 3.95 10-4
- Dairy Kd = 1.4 10-4
From the value pH depends on the quantitative ratio of primary and secondary fermentation products, the wine's tendency to oxidation, crystalline and biological turbidity, susceptibility to defects and resistance to wine diseases.
Examples
A simple explanation of the logarithmic relationship. Solution with pH= 3 is ten times more acidic than a solution with pH= 4. Or, for a more practical example, wine with pH= 3.2 25% more acidic than wine with pH= 3.3.
If it is necessary to correct the acidity of the wine, winemakers add a mixture of 1.9 g/l of lactic acid and 2.27 g/l of tartaric (dioxisuccinic or tartaric) acid. This makes it possible to reduce pH approximately by 0.1 (range 3 to 4).
And if, for example, the wine turned out with pH = 3.7 and the winemaker wants to bring it to pH = 3.5, he will double this “dose”.
ValuepHfor some products
The table below shows the acidity values of some common foods and pure water at different temperatures:
| Product | Acidity, pH |
| Lemon juice | 2,1 |
| Wine, approx. | 3,5 |
| Tomato juice | 4,1 |
| Orange juice | 4,2 |
| Black coffee | 5,0 |
| Pure water at 100°C | 6,13 |
| Pure water at 50°C | 6,63 |
| Fresh milk | 6,68 |
| Pure water at 22°C | 7,0 |
| Pure water at 0°C | 7,48 |
Volatile acidity
Volatile acidity, or VA for short, is that portion of the acids in wine that can be detected by the nose.
Unlike those acids that are palpable to the taste (as we talked about above).
Volatile acidity, or in other words, souring of wine, is one of the most common defects. Its main culprits are acetic acid (smells like vinegar) and its ester, ethyl acetate (smells like nail polish).
The bacteria responsible for volatile acidity thrive under conditions of low acidity and high sugar content. In small concentrations, volatile acidity gives the wine a piquancy. And when the threshold is exceeded, the vinegar-lacquer component clogs useful aromas and spoils the taste of wine.
The hydrogen index - pH - is a measure of the activity (in the case of dilute solutions it reflects the concentration) of hydrogen ions in a solution, quantitatively expressing its acidity, calculated as a negative (taken with the opposite sign) decimal logarithm of the activity of hydrogen ions, expressed in moles per liter.
pH = – lg
This concept was introduced in 1909 by the Danish chemist Sorensen. The indicator is called pH, after the first letters of the Latin words potentia hydrogeni - the strength of hydrogen, or pondus hydrogenii - the weight of hydrogen.
The reciprocal pH value has become somewhat less widespread - an indicator of the basicity of the solution, pOH, equal to the negative decimal logarithm of the concentration in the solution of OH ions:
pOH = – lg
In pure water at 25 ° C, the concentrations of hydrogen ions () and hydroxide ions () are the same and amount to 10 -7 mol / l, this directly follows from the water autoprotolysis constant K w, which is otherwise called the ion product of water:
K w \u003d \u003d 10 -14 [mol 2 / l 2] (at 25 ° C)
pH + pOH = 14
When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. When an acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions decreases accordingly, when a base is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases. When > they say that the solution is acidic, and when > - alkaline.
pH determination
Several methods are widely used to determine the pH value of solutions.
1) The pH value can be approximated with indicators, accurately measured with a pH meter, or determined analytically by performing an acid-base titration.
For a rough estimate of the concentration of hydrogen ions, acid-base indicators are widely used - organic dye substances, the color of which depends on the pH of the medium. The most famous indicators include litmus, phenolphthalein, methyl orange (methyl orange) and others. Indicators can exist in two differently colored forms, either acidic or basic. The color change of each indicator occurs in its acidity range, usually 1-2 units (see Table 1, lesson 2).
To extend the working range of pH measurement, the so-called universal indicator is used, which is a mixture of several indicators. The universal indicator consistently changes color from red through yellow, green, blue to purple when moving from an acidic to an alkaline region. Determination of pH by the indicator method is difficult for cloudy or colored solutions.
2) The analytical volumetric method - acid-base titration - also gives accurate results for determining the total acidity of solutions. A solution of known concentration (titrant) is added dropwise to the test solution. When they are mixed, a chemical reaction takes place. The equivalence point - the moment when the titrant is exactly enough to completely complete the reaction - is fixed using an indicator. Further, knowing the concentration and volume of the added titrant solution, the total acidity of the solution is calculated.
The acidity of the environment is important for many chemical processes, and the possibility of the occurrence or the result of a particular reaction often depends on the pH of the environment. To maintain a certain pH value in the reaction system in laboratory studies or in production, buffer solutions are used that allow you to maintain a practically constant pH value when diluted or when small amounts of acid or alkali are added to the solution.
The pH value is widely used to characterize the acid-base properties of various biological media (Table 2).
The acidity of the reaction medium is of particular importance for biochemical reactions occurring in living systems. The concentration of hydrogen ions in a solution often affects the physicochemical properties and biological activity of proteins and nucleic acids, therefore, maintaining acid-base homeostasis is a task of exceptional importance for the normal functioning of the body. Dynamic maintenance of the optimal pH of biological fluids is achieved through the action of buffer systems.
3) The use of a special device - a pH meter - allows you to measure pH in a wider range and more accurately (up to 0.01 pH units) than using indicators, is convenient and highly accurate, allows you to measure the pH of opaque and colored solutions and therefore widely used.
Using a pH meter, the concentration of hydrogen ions (pH) is measured in solutions, drinking water, food products and raw materials, environmental objects and production systems for continuous monitoring of technological processes, including in aggressive environments.
A pH meter is indispensable for hardware monitoring of the pH of uranium and plutonium separation solutions, when the requirements for the correctness of equipment readings without its calibration are extremely high.
The device can be used in stationary and mobile laboratories, including field laboratories, as well as clinical diagnostic, forensic, research, industrial, including meat and dairy and baking industries.
Recently, pH meters have also been widely used in aquarium farms, household water quality control, agriculture (especially in hydroponics), and also for monitoring health diagnostics.
Table 2. pH values for some biological systems and other solutions
|
System (solution) | |
|
Duodenum | |
|
gastric juice | |
|
human blood | |
|
Muscle | |
|
pancreatic juice | |
|
cell protoplasm | |
|
Small intestine | |
|
Sea water | |
|
Chicken egg white | |
|
Orange juice | |
|
Tomato juice | |
pH value and its influence on the quality of drinking water.
What is pH?
pH("potentia hydrogeni" - the strength of hydrogen, or "pondus hydrogenii" - the weight of hydrogen) is a unit of measurement of the activity of hydrogen ions in any substance, quantitatively expressing its acidity.
This term appeared at the beginning of the twentieth century in Denmark. The pH index was introduced by the Danish chemist Soren Petr Lauritz Sorensen (1868-1939), although his predecessors also have statements about a certain “power of water”.
Hydrogen activity is defined as the negative decimal logarithm of the concentration of hydrogen ions, expressed in moles per liter:
pH = -log
For simplicity and convenience, pH was introduced in the calculations. pH is determined by the quantitative ratio of H+ and OH- ions in water, which are formed during the dissociation of water. It is customary to measure the pH level on a 14-digit scale.
If the water has a reduced content of free hydrogen ions (pH greater than 7) compared to hydroxide ions [OH-], then the water will have alkaline reaction, and with an increased content of H + ions (pH less than 7) - acid reaction. In perfectly pure distilled water, these ions will balance each other.
acid environment: >
neutral environment: =
alkaline environment: >
When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. In neutral water, the pH is 7.
When various chemicals are dissolved in water, this balance changes, which leads to a change in the pH value. When acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions decreases accordingly, when alkali is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases.
The pH indicator reflects the degree of acidity or alkalinity of the environment, while "acidity" and "alkalinity" characterize the quantitative content of substances in water that can neutralize alkalis and acids, respectively. As an analogy, we can give an example with temperature, which characterizes the degree of heating of a substance, but not the amount of heat. By dipping our hand into the water, we can tell whether the water is cool or warm, but at the same time we will not be able to determine how much heat is in it (i.e., relatively speaking, how long this water will cool down).
pH is considered one of the most important indicators of drinking water quality. It shows the acid-base balance and influences how chemical and biological processes will proceed. Depending on the pH value, the rate of chemical reactions, the degree of corrosiveness of water, the toxicity of pollutants, etc. can change. Our well-being, mood and health directly depend on the acid-base balance of the environment of our body.
Modern man lives in a polluted environment. Many people buy and consume food made from semi-finished products. In addition, almost every person is exposed to stress on a daily basis. All this affects the acid-base balance of the body's environment, shifting it towards acids. Tea, coffee, beer, carbonated drinks lower the pH in the body.
It is believed that an acidic environment is one of the main causes of cell destruction and tissue damage, the development of diseases and the aging process, and the growth of pathogens. In an acidic environment, building material does not reach the cells, the membrane is destroyed.
Outwardly, the state of the acid-base balance of a person's blood can be judged by the color of his conjunctiva in the corners of his eyes. With an optimal acid-base balance, the color of the conjunctiva is bright pink, but if a person has an increased alkalinity of the blood, the conjunctiva acquires a dark pink color, and with an increase in acidity, the color of the conjunctiva becomes pale pink. Moreover, the color of the conjunctiva changes already 80 seconds after the use of substances that affect the acid-base balance.
The body regulates the pH of internal fluids, maintaining the values at a certain level. The acid-base balance of the body is a certain ratio of acids and alkalis that contributes to its normal functioning. Acid-base balance depends on maintaining relatively constant proportions between intercellular and intracellular waters in the tissues of the body. If the acid-base balance of fluids in the body is not constantly maintained, normal functioning and the preservation of life will be impossible. Therefore, it is important to control what you consume.

Acid-base balance is our indicator of health. The more acidic we are, the sooner we age and the more we get sick. For the normal functioning of all internal organs, the pH level in the body must be alkaline, in the range from 7 to 9.
The pH inside our body is not always the same - some parts are more alkaline and some are more acidic. The body regulates and maintains pH homeostasis only in certain cases, such as blood pH. The pH level of the kidneys and other organs, the acid-base balance of which is not regulated by the body, is affected by the food and drinks that we consume.
blood pH
The blood pH level is maintained by the body in the range of 7.35-7.45. The normal pH of human blood is 7.4-7.45. Even a slight deviation in this indicator affects the ability of the blood to carry oxygen. If the pH of the blood rises to 7.5, it carries 75% more oxygen. With a decrease in blood pH to 7.3, it is already difficult for a person to get out of bed. At 7.29, he can fall into a coma, if the blood pH drops below 7.1, the person dies.
Blood pH must be maintained in a healthy range, so the body uses organs and tissues to keep it constant. As a consequence, the pH level of the blood does not change due to the consumption of alkaline or acidic water, but the tissues and organs of the body used to adjust the pH of the blood change their pH.
kidney pH
The pH parameter of the kidneys is influenced by water, food, and metabolic processes in the body. Acidic foods (such as meats, dairy, etc.) and drinks (sweetened sodas, alcoholic beverages, coffee, etc.) lead to low pH levels in the kidneys because the body excretes excess acidity through urine. The lower the pH of the urine, the harder it is for the kidneys to work. Therefore, the acid load on the kidneys from such foods and drinks is called the potential acid-renal load.
The use of alkaline water benefits the kidneys - there is an increase in the pH level of the urine, the acid load on the body is reduced. Increasing the pH of the urine raises the pH of the body as a whole and rids the kidneys of acidic toxins.
stomach pH
An empty stomach contains no more than a teaspoon of stomach acid produced in the last meal. The stomach produces acid as needed when eating food. The stomach does not release acid when a person drinks water.
It is very helpful to drink water on an empty stomach. The pH increases at the same time to the level of 5-6. An increased pH will have a mild antacid effect and lead to an increase in beneficial probiotics (beneficial bacteria). Increasing the pH of the stomach raises the pH of the body, which leads to healthy digestion and relief from the symptoms of indigestion.
subcutaneous fat pH
The fatty tissues of the body have an acidic pH because excess acids are deposited in them. The body has to store acid in fatty tissues when it cannot be removed or neutralized in other ways. Therefore, the shift in the pH of the body to the acid side is one of the factors of excess weight.
The positive effect of alkaline water on body weight is that alkaline water helps to remove excess acid from the tissues, as it helps the kidneys to work more efficiently. This helps to control weight, since the amount of acid that the body must "store" is greatly reduced. Alkaline water also enhances the results of a healthy diet and exercise by helping the body deal with the excess acid produced by fatty tissue during weight loss.
Bones
Bones have an alkaline pH as they are mostly made up of calcium. Their pH is constant, but if the blood needs pH adjustment, calcium is taken from the bones.
The benefit that alkaline water brings to the bones is to protect them by reducing the amount of acid that the body has to deal with. Studies have shown that drinking alkaline water reduces bone resorption - osteoporosis.
liver pH
The liver has a slightly alkaline pH, which is affected by both food and drink. Sugar and alcohol must be broken down in the liver, and this leads to excess acid.
The benefits of alkaline water for the liver are the presence of antioxidants in such water; it has been found that alkaline water enhances the work of two antioxidants located in the liver, which contribute to more effective blood purification.
body pH and alkaline water
Alkaline water allows the parts of the body that maintain the pH of the blood to work more efficiently. Increasing the pH level in the parts of the body responsible for maintaining blood pH will help these organs stay healthy and function efficiently.
Between meals, you can help your body balance its pH by drinking alkaline water. Even a small increase in pH can have a huge impact on health.
According to research by Japanese scientists, the pH of drinking water, which is in the range of 7-8, increases the life expectancy of the population by 20-30%.
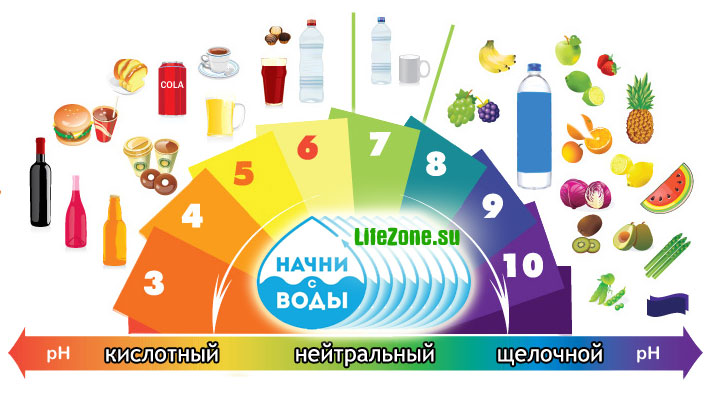
Depending on the pH level, water can be divided into several groups:
strongly acidic waters< 3
acidic waters 3 - 5
slightly acidic waters 5 - 6.5
neutral waters 6.5 – 7.5
slightly alkaline waters 7.5 - 8.5
alkaline waters 8.5 - 9.5
highly alkaline waters > 9.5
Typically, the pH level of drinking tap water is within the range at which it does not directly affect the consumer quality of water. In river waters pH is usually within 6.5-8.5, in atmospheric precipitation 4.6-6.1, in swamps 5.5-6.0, in sea waters 7.9-8.3.
WHO does not offer any medically recommended value for pH. It is known that at low pH, water is highly corrosive, and at high levels (pH>11), water acquires a characteristic soapiness, an unpleasant odor, and can cause eye and skin irritation. That is why for drinking and domestic water, the pH level in the range from 6 to 9 is considered optimal.
|
|||||||||||||||||||||||||||||||||||||||||||||||



Interesting to know: The German biochemist OTTO WARBURG, who was awarded the Nobel Prize in Physiology or Medicine in 1931, proved that the lack of oxygen (an acidic pH<7.0) в тканях приводит к изменению нормальных клеток в злокачественные.
The scientist found that cancer cells lose their ability to develop in an environment saturated with free oxygen with a pH value of 7.5 and higher! This means that when the fluids in the body become acidic, the development of cancer is stimulated.
His followers in the 60s of the last century proved that any pathogenic flora loses its ability to multiply at pH = 7.5 and above, and our immune system can easily cope with any aggressors!
To preserve and maintain health, we need proper alkaline water (pH=7.5 and above). This will allow you to better maintain the acid-base balance of body fluids, since the main living environments have a slightly alkaline reaction.
Already in a neutral biological environment, the body can have an amazing ability to heal itself.

Don't know where to get correct water ? I'll prompt!

Note:
Pressing the button " To know» does not lead to any financial expenses and obligations.
You are only get information about the availability of the right water in your area,
as well as get a unique opportunity to become a member of the club of healthy people for free
and get a 20% discount on all offers + cumulative bonus.

Join the international health club Coral Club, get a FREE discount card, the opportunity to participate in promotions, a cumulative bonus and other privileges!

Hydrogen index, pH (pronounced “peash”, English pronunciation of English pH - piː "eɪtʃ," pee ") - a measure of activity (in very dilute solutions it is equivalent to concentration) of hydrogen ions in a solution, and quantitatively expressing its acidity, is calculated as the negative (reverse sign) logarithm of the tenth logarithm of the activity of hydrogen ions, expressed in moles per liter: Story This concept was introduced in 1909 by the Danish chemist Sorensen. The indicator is called pH, after the first letters of the Latin words potentia hydrogeni - the strength of hydrogen, or pondus hydrogeni - the weight of hydrogen. In general, in chemistry, the combination pX is usually used to denote a value equal to −lg X, and the letter H in this case denotes the concentration of hydrogen ions (H +), or, more precisely, the thermodynamic activity of hydronium ions. Equations relating pH and pOH pH value output In pure water at 25 ° C, the concentrations of hydrogen ions () and hydroxide ions () are the same and amount to 10 −7 mol / l, this directly follows from the definition of the ion product of water, which is equal to and is 10 −14 mol² / l² (at 25°C). When the concentrations of both types of ions in a solution are the same, the solution is said to be neutral. When acid is added to water, the concentration of hydrogen ions increases, and the concentration of hydroxide ions decreases accordingly, when a base is added, on the contrary, the content of hydroxide ions increases, and the concentration of hydrogen ions decreases. When > they say that the solution is acidic, and when > - alkaline. For convenience of presentation, in order to get rid of the negative exponent, instead of the concentrations of hydrogen ions, their decimal logarithm, taken with the opposite sign, is used, which is actually the hydrogen indicator - pH. pOH The reciprocal pH value has become somewhat less widespread - an indicator of the basicity of the solution, pOH, equal to the negative decimal logarithm of the concentration in the solution of OH - ions: as in any aqueous solution at 25 °C, it is obvious that at this temperature: pH values in solutions of different acidity
- Contrary to popular belief, pH can vary not only in the range from 0 to 14, but can also go beyond these limits. For example, at a concentration of hydrogen ions = 10 −15 mol / l, pH = 15, at a concentration of hydroxide ions of 10 mol / l pOH = −1.
|
- For a rough estimate of the concentration of hydrogen ions, acid-base indicators are widely used - organic dye substances, the color of which depends on the pH of the medium. The most famous indicators include litmus, phenolphthalein, methyl orange (methyl orange) and others. Indicators can exist in two differently colored forms, either acidic or basic. The color change of each indicator occurs in its acidity range, usually 1–2 units.
- To extend the working range of pH measurement, the so-called universal indicator is used, which is a mixture of several indicators. The universal indicator consistently changes color from red through yellow, green, blue to purple when moving from an acidic to an alkaline region. Determination of pH by the indicator method is difficult for cloudy or colored solutions.
- The use of a special device - a pH meter - allows you to measure pH in a wider range and more accurately (up to 0.01 pH units) than with indicators. The ionometric method for determining pH is based on measuring the EMF of a galvanic circuit with a millivoltmeter-ionometer, including a special glass electrode, the potential of which depends on the concentration of H + ions in the surrounding solution. The method is convenient and highly accurate, especially after calibrating the indicator electrode in a selected pH range, allows you to measure the pH of opaque and colored solutions, and therefore is widely used.
- Analytical volumetric method - acid-base titration - also gives accurate results for determining the acidity of solutions. A solution of known concentration (titrant) is added dropwise to the test solution. When they are mixed, a chemical reaction takes place. The equivalence point - the moment when the titrant is exactly enough to completely complete the reaction - is fixed using an indicator. Further, knowing the concentration and volume of the added titrant solution, the acidity of the solution is calculated.
- Effect of Temperature on pH Values
The degree of acid-base indicators, determined by the concentration of hydrogen ions, forms the pH parameters, which are normally 6-9 units for drinking water, according to the rules of SanPinN. According to this indicator, Russian standards almost do not differ from the EU directive - 6.50-9.50 and from the requirements of the US Environmental Protection Agency (USEPA) - 6.50-8.50.
At the same time, the pH norms of water intended for various industrial needs differ from the pH norms of water for drinking. For example:
- in hydroponics, solutions with a level of 5.50-7.50 are used and this range is divided into narrower segments depending on the specific plant species,
- in public pools this standard is 7.20-7.40; in private wider - 7.20-7.60; according to DIN 19643-1 - 6.50-7.60,
- in the production of beer, a water base with indicators of 6.00-6.50 is used,
- for soft drinks - 3.00-6.00,
- for export vodka, the indicator depends on the hardness of the process water - and is equal to 7 with hardness from 0 to 0.60 meq/l and 6.50 - at 0.61-1.2 meq/l; in “domestic market” vodkas – pH<7,80,
- in chemical fiber production - 7.00-8.00,
- in dyeing and finishing - 6.50-8.50,
- in heat supply systems, the parameter is indicated at a temperature of +25ºС and is in the range of 7.00-8.50 for open systems and within 7.00-11.00 for closed ones,
- in power engineering and steam boilers - not less than 8.50,
- in cooling systems: for circulating and additional water - 6.50-8.50, in the circulation cold circuit - 6.50-8.20, hot circuit - 6.80-8.00, etc.
Determining the level and dependences of pH
The scale for determining the nature of the acid-base environment consists of 14 units, where the median value of pH=7 is considered neutral. With a shift along this scale to the beginning (to zero), the solutions become acidic. When shifted to the end - the nature of the alkaline. Most often, such a dependence is reflected in tables with frequent gradation:

For comparison, according to GOST 6709-96, the pH distillate can have values in the range of 5.40-6.60.
Since the concentration of hydrogen ions is low (for a neutral medium it is seven zeros after the decimal point), the indicator is expressed in a more familiar form as a negative decimal logarithm. In tables, “pH, units” is usually written as units of measurement. or µg/l (micrograms per litre).
The pH value differs from the total alkalinity (water alkalinity), which, expressed in mg-eq/l, is determined by the sum of hydroxyl ions/anions of weak acids in water. Low alkalinity provokes a sharp change in pH under the influence of external factors.
In natural waters, pH, in most cases, is in the range of 6.50-8.50, reflecting the dependence on the ratios of free carbon dioxide on the one hand, and bicarbonate ion on the other. In swamp waters, pH values are lower and shift towards acidity. Often this parameter becomes an indicator of pollution in open water bodies, demonstrating the presence of effluents with a high content of acid or alkali.
With intensive photosynthesis, which is observed in summer, the level of the indicator can rise to 8.50-9.00 units. Also, the values of the parameter are affected by the concentration of carbonates subject to the hydrolysis of salts, hydroxides, humic substances, etc.
The importance of pH in everyday life
Japanese scientists conducted comparative studies of consumers in areas where they use drinking water with pH values shifted either towards acidity or towards alkalinity. They concluded that in areas where this indicator is above average, people live 20-30% longer than the average life expectancy in the country. As a presumed reason, the greater "comfort" of acidic waters for the development of pathological microflora is called.
Due to the fact that tap water really has a significant impact on human health, some technical accessories that come into contact with it are beginning to be advertised as agents that can change the chemical properties of water. For example, http://water-save.com/ savers are described as devices that "enrich the water with weak ions that activate the metabolism." In fact, only the economic, but not the “healing” effect of the installation of the economizer is reliably confirmed.
This, however, does not negate the value of the pH parameter for the organism. Each environment - including the various environments of the human body - has its own "pH-guides":
- saliva - 6.8-7.4 (with a high rate of salivation - 7.8),
- tears - 7.3-7.5,
- blood - 7.43,
- lymph - 7.5,
- urine - 5.5 (range 5.0-7.5), etc.

To visually demonstrate the acid-base state of various media, there are tables in which the values are arranged in ascending order:



